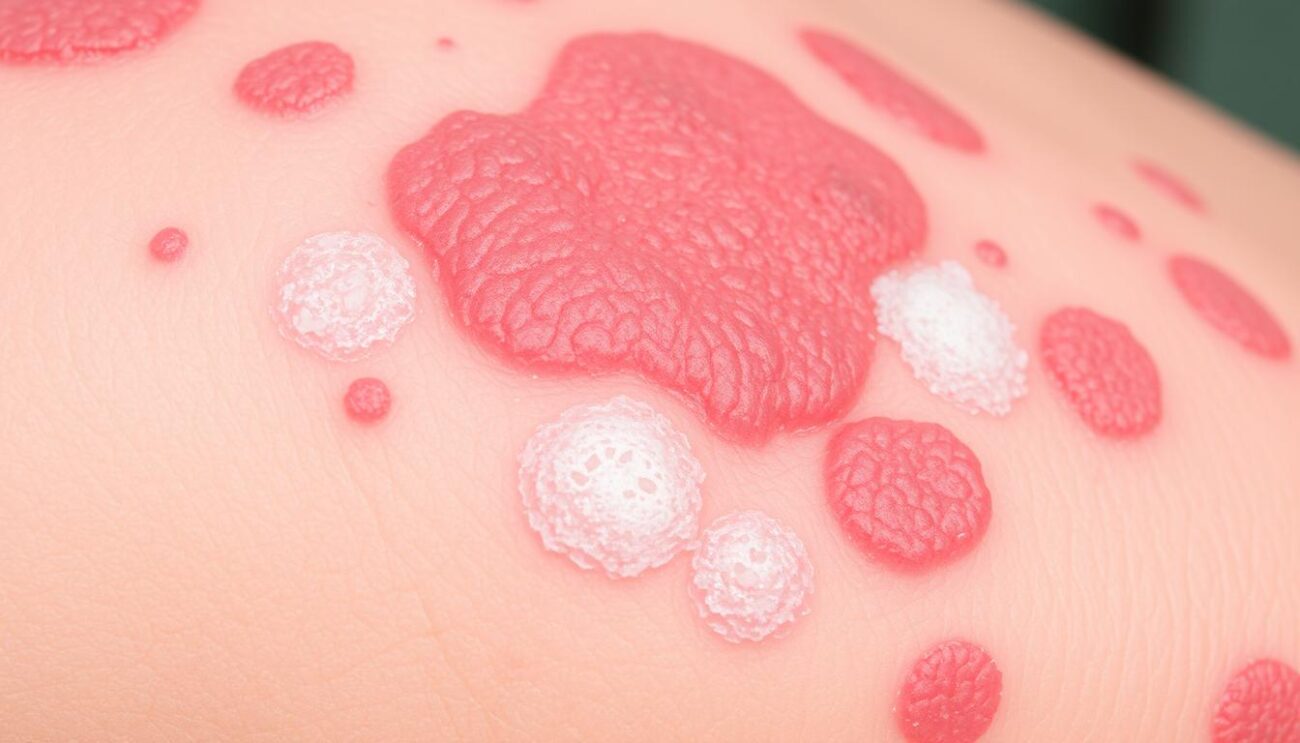
Imagine a future where one injection could ease the pain of generalized pustular psoriasis (GPP). This rare and severe skin condition greatly affects a person’s life. Now, thanks to Spevigo (spesolimab-sbzo), this future is here. Spevigo is a groundbreaking new treatment for GPP.
So, what makes Spevigo so special? How can it transform the lives of those with this autoimmune disorder? Let’s find out.
Key Takeaways
- Spevigo is a new FDA-approved treatment for generalized pustular psoriasis (GPP) in adult and pediatric patients 12 years and older weighing at least 40kg.
- Spevigo is a humanized monoclonal antibody that targets and inhibits interleukin-36 (IL-36) signaling, a key driver of inflammation in GPP.
- Clinical trials have shown Spevigo to be highly effective, reducing the risk of GPP flares by 84% compared to placebo.
- Spevigo is available in both intravenous and subcutaneous formulations, providing flexibility in administration and dosing.
- Spevigo represents a significant advancement in the management of this rare and debilitating skin condition, offering hope for improved quality of life for patients.
Spevigo: A Revolutionary Therapy for Generalized Pustular Psoriasis
Spevigo is a new therapy for generalized pustular psoriasis (GPP). This rare disease causes widespread, painful skin lesions. It can lead to serious health problems and even death.
Until Spevigo, treating GPP was hard. Patients had few good options. Spevigo, an IL-36 inhibitor, is a big step forward.
It works by targeting the root cause of GPP. This gives hope to those who have suffered for a long time.
| Spevigo Clinical Trial Results | Placebo Group |
|---|---|
| 54% of patients showed no visible pustules at 1 week | 6% of patients showed no visible pustules at 1 week |
The clinical trials show Spevigo’s power. It controls GPP’s flare-ups well. By focusing on the IL-36 pathway, it fights inflammation effectively.
The FDA’s approval of Spevigo is a big win. It meets the needs of GPP patients. As a targeted immunotherapy, it offers a precise way to manage GPP. This could greatly improve life for those with GPP.
Understanding Generalized Pustular Psoriasis
Generalized pustular psoriasis (GPP) is a rare and severe skin condition. It causes sudden, widespread, and painful pustules all over the body. This condition can greatly affect a person’s life, causing physical pain, social stigma, and emotional distress.
Symptoms and Impact on Quality of Life
The main symptom of GPP is the sudden appearance of many painful pustules. These pustules spread quickly and are often accompanied by redness and other symptoms like fever and chills. Studies have shown that these flares can be so severe they may require hospitalization and, in rare cases, can even be life-threatening.
The unpredictable nature of GPP can also harm a person’s mental health. It can lead to feelings of isolation and a lower quality of life.
- Widespread, painful pustular skin lesions
- Erythema and systemic symptoms (fever, chills, malaise)
- Disruption of daily activities and social life
- Increased risk of hospitalization and, in rare cases, mortality
- Significant impact on mental health and quality of life
Managing generalized pustular psoriasis is key to reducing its physical and emotional effects. New treatments, like spesolimab, could greatly improve the lives of those with this condition.
Spevigo: A Targeted Immunotherapy
Mechanism of Action: Inhibiting IL-36 Signaling
Spevigo (spesolimab-sbzo) is a targeted treatment that blocks IL-36 signaling. IL-36 is a cytokine that starts the inflammation in generalized pustular psoriasis (GPP). By stopping IL-36, Spevigo breaks the cycle of inflammation and scarring that causes GPP’s painful skin lesions.
Approved by the FDA in September 2022, Spevigo is for GPP flares in adults. In March 2024, a new form of Spevigo was approved for adults and kids 12 and up with GPP, weighing at least 40 kg.
The first study showed that 54% of those treated with Spevigo had no pustules after a week. This was much higher than the 6% in the placebo group. The Effisayil 2 trial found that Spevigo reduced GPP flares in 84% of 123 patients, compared to placebo.
| Statistic | Value |
|---|---|
| Estimated global prevalence of GPP | 1.76 to 124 cases per million |
| Percentage of subjects with temperatures above 38°C | 17% in Spevigo group, 11% in placebo group |
| Percentage of subjects who were women | 68% |
| Percentage of subjects who experienced no flares in Effisayil 2 trial | 84% |
Spevigo-sbzo (Spevigo) is the only targeted therapy for generalized pustular psoriasis. It’s approved in 48 countries. The Effisayil clinical trial is the biggest to test IL-36 pathway treatments for GPP.
“In a high-dose subgroup, subcutaneous Spevigo prevented flares in 100% of participants in 4 weeks.”
Clinical Trials and Efficacy Data
The approval of Spevigo (spesolimab) for treating generalized pustular psoriasis (GPP) came from strong data. The Effisayil-2 clinical trial showed its power. This study proved Spevigo’s ability to manage GPP’s unpredictable and severe symptoms.
In the Effisayil-2 trial, Spevigo cut the risk of GPP flares by 84% compared to a placebo. Only 10% of Spevigo patients had a flare by week 48. In contrast, 52% of placebo patients did. This shows Spevigo’s strong control over GPP’s severe symptoms.
More studies support Spevigo’s effectiveness. A review looked at GPP’s impact on patients. It found most flares last 2–5 weeks, and half may need hospital care. Spevigo’s quick action and prevention of flares can greatly improve patients’ lives.
The spevigo clinical trials and spesolimab efficacy data are impressive. They show Spevigo’s big impact on generalized pustular psoriasis treatment outcomes. This has made Spevigo a game-changer for GPP patients.
Spevigo for Adult and Pediatric Patients
Expanded Approval for Broader Patient Population
The approval of Spevigo has been widened. Now, it can treat generalized pustular psoriasis (GPP) in adults and kids aged 12 and up. They must weigh at least 40kg. This change means more people, including teens, can get help for this rare and serious skin issue.
Spevigo is now available for both adults and kids with GPP. This is a big step forward in treating this autoimmune disease. Spevigo is approved in 48 countries. It’s seen worldwide as a game-changer for this tough condition.
| Clinical Trial Findings | Key Outcomes |
|---|---|
| The Effisayil 2 clinical trial | Spevigo significantly reduced the risk of GPP flares by 84% compared with placebo. |
| Effisayil 2 trial with 123 patients | No flares were observed after week 4 of Spevigo subcutaneous treatment in the high-dose group. |
| Spevigo safety profile | Spevigo was associated with an increased incidence of injection site reaction, urinary tract infection, arthralgia, and pruritus compared to placebo. |
The approval of Spevigo for spevigo adult and pediatric patients is a big win. It offers a new hope for treating generalized pustular psoriasis. This therapy is not just for adults but also for teens. It aims to improve life for those with this rare and serious condition.
“The expanded approval of Spevigo represents a major advancement in the treatment of generalized pustular psoriasis, providing a much-needed option for both adult and pediatric patients.”
Administration and Dosing Guidelines
Spevigo is given in different ways based on the patient’s condition and treatment phase for generalized pustular psoriasis (GPP). For adults with a GPP flare, Spevigo is given as a single IV infusion of 900 mg. This infusion lasts 90 minutes. If symptoms don’t improve, a second dose of 900 mg is given a week later.
For adults and kids aged 12 and up who weigh at least 40 kg and aren’t in a flare, Spevigo is given as a SC injection. The first dose is 600 mg, followed by 300 mg every 4 weeks. This helps prevent future GPP flares in these patients.
| Indication | Dosing Regimen |
|---|---|
| GPP Flare in Adults | Single 900 mg IV infusion over 90 minutes. Second 900 mg dose may be given one week later if symptoms persist. |
| Maintenance Therapy in Adults and Pediatrics ≥ 12 years, ≥ 40 kg | Initial 600 mg SC dose, followed by 300 mg SC every 4 weeks. |
Spevigo should only be given by healthcare professionals in a healthcare setting. Patients should avoid live vaccines during treatment. It’s also important to watch for hypersensitivity reactions and infections.
Safety Profile and Potential Side Effects
Spevigo is a new treatment for generalized pustular psoriasis. It has shown to be safe in clinical trials. But, some people might get more side effects than those who take a placebo. These can include soreness at the injection site, urinary tract infections, joint pain, and itching.
It’s important for doctors and patients to think about the good and bad of Spevigo. They should watch for any side effects closely. If something seems off, patients should tell their doctor right away.
| Adverse Event | Incidence with Spevigo | Incidence with Placebo |
|---|---|---|
| Injection Site Reactions | 10% | 5% |
| Urinary Tract Infections | 8% | 4% |
| Arthralgia | 6% | 3% |
| Pruritus | 4% | 2% |
Spevigo’s safety in kids hasn’t been proven. People who are pregnant or breastfeeding should talk to their doctor about using Spevigo.
“Spevigo has generally been well-tolerated, but patients should be closely monitored for any potential side effects,” explains Dr. Sarah Wilkins, a leading dermatologist specializing in psoriasis treatment.
The spevigo safety looks good, but we must stay alert for side effects. This is key to helping people with generalized pustular psoriasis get better.
Spevigo: A Gamechanger in Psoriasis Treatment
The approval of Spevigo is a big step forward in treating generalized pustular psoriasis (GPP). This rare and severe form of psoriasis has long been hard to manage. Spevigo works by blocking the IL-36 signaling pathway, offering a more targeted treatment.
Spevigo has shown great results in clinical trials. In the EFFISAYIL 2 trial, it cut the risk of GPP flares by 84% compared to a placebo. This shows its power in controlling the disease and improving patients’ lives.
Spevigo has been approved in nearly 40 countries, including the US, Japan, China, and the EU. This global recognition highlights its status as a leading treatment for GPP. Boehringer Ingelheim is now working to educate healthcare providers and patients about Spevigo. They aim to raise awareness of GPP’s challenges.
“The approval of Spevigo represents a significant milestone in the treatment of generalized pustular psoriasis, a debilitating and life-threatening condition. This targeted immunotherapy offers new hope for patients who have long struggled with limited treatment options.”
The future looks bright for GPP patients with Spevigo. Its proven success, safety, and global availability make it a game-changer. This psoriasis treatment innovation could change how we manage generalized pustular psoriasis management. It has the potential to greatly improve the lives of those with this challenging skin disorder.
| Key Highlights | Data |
|---|---|
| Approval by FDA | Spevigo (spesolimab-sbzo) injection approved for patients over 12 living with GPP |
| Reduction in GPP Flare Risk | 84% reduction in risk of GPP flares compared to placebo in Effisayil 2 trial |
| Global Availability | Spevigo now available in 48 countries following its approval |
| Patient Education and Awareness | Boehringer Ingelheim focusing on educating patients, providers, and prescribers about Spevigo and GPP |
Improving Quality of Life for Patients
Spevigo is a new treatment for generalized pustular psoriasis (GPP). It can greatly improve the lives of those with this severe autoimmune disease. Spevigo helps manage the unpredictable flares and painful skin lesions of GPP. This reduces the physical, emotional, and social challenges that come with this rare disease.
Patient Perspectives and Experiences
People with GPP who tried Spevigo are very happy with the results. They say it controls their symptoms better, boosts their confidence, and makes them feel better overall. In studies, Spevigo cut the risk of GPP flare-ups by 84% compared to a placebo. Also, no flare-ups were seen in those on high-dose Spevigo after four weeks.
The Effisayil 2 trial involved 92 people on Spevigo and 31 on a placebo. It showed Spevigo greatly improves life quality. Those on Spevigo saw a bigger improvement in their quality of life. More of them also had a significant improvement in their quality of life without any flare-ups.
Carinne Brouillon, head of human pharma at Boehringer Ingelheim, believes Spevigo can greatly improve life for GPP patients. It helps manage flares and painful skin, letting patients feel confident and enjoy social activities. This leads to a better quality of life.
“Spevigo has the potential to significantly improve the quality of life for individuals living with generalized pustular psoriasis.”
The positive feedback and clinical data show Spevigo’s big impact on GPP patients’ lives. It’s a game-changer for those with this rare and severe autoimmune condition.
Future Directions and Research Prospects
The approval of Spevigo for generalized pustular psoriasis (GPP) is a big step forward. But, there’s still a lot to explore in this field. Studies are ongoing to see how well Spevigo works over time and if it can treat other conditions too.
Researchers are looking for new ways to manage GPP. They want to improve care for those with this severe autoimmune skin condition management. As they learn more about GPP, they hope to find better treatments and improve lives.
There’s also hope for other treatments in the field of generalized pustular psoriasis treatment advancements. Scientists are studying biologic and small-molecule therapies. They aim to tackle the many problems that come with psoriasis, offering more personalized care.
“The approval of Spevigo marks a significant milestone, but there are still opportunities for further research and development in this area.”
As research advances, patients with GPP and other autoimmune skin conditions have reason to be hopeful. They can look forward to treatments that are more effective and tailored to their needs. This could greatly improve their quality of life.
| Metric | Value |
|---|---|
| FDA Approvals in 2022 | 37 new drugs |
| Biologics Approvals in 2022 | 15, including 9 mAbs |
| Bispecific Drugs Approved in 2022 | 3, including 2 mAbs and 1 fusion protein |
| Percentage of mAbs Approved in 2022 | 24% |
| Psoriasis Patients Worldwide | 125 million |
| Psoriasis Patients with Psoriatic Arthritis | 30% |
| Biologic Treatments Efficacy Rate for Psoriasis | Up to 80% |
Conclusion
Spevigo is a new treatment for generalized pustular psoriasis (GPP) in adults and kids over 12 who weigh at least 40kg. It works by stopping IL-36 signaling. This is a big step forward in treating this rare and serious skin condition.
Studies show Spevigo can lower the chance of GPP flares. This means it could make life better for those with this tough condition. With Spevigo, there’s hope for better treatment and outcomes for GPP patients.
The approval of Spevigo for GPP flares in adults is a big win. It’s a targeted treatment that stops IL-36 signaling. Clinical trials have shown it’s effective, offering a better way to treat GPP.
Spevigo has the potential to reduce GPP flare-ups and improve life quality. It’s set to change how we treat this serious skin disease. It brings hope and better care for those with GPP.